MasterControl Registrations for eCTD
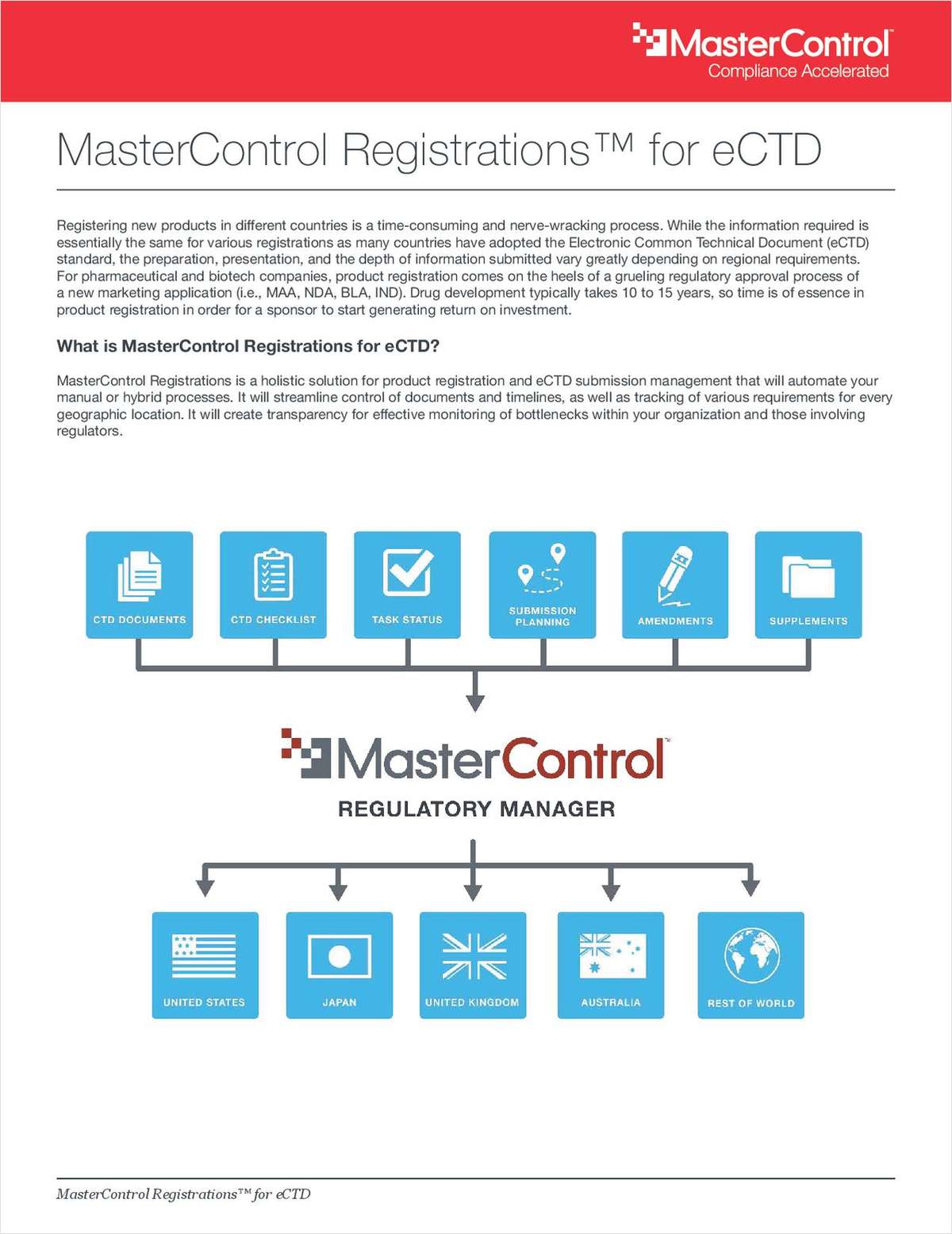
Registering new products in different countries is a time-consuming and nerve-wracking process.
While the information required is essentially the same for various registrations as many countries have adopted the Electronic Common Technical Document (eCTD) standard, the preparation, presentation, and the depth of information submitted vary greatly depending on regional requirements. For pharmaceutical and biotech companies, product registration comes on the heels of a grueling regulatory approval process of a new marketing application (i.e., MAA, NDA, BLA, IND). Drug development typically takes 10 to 15 years, so time is of essence in product registration in order for a sponsor to start generating return on investment.





