MasterControl Registrations for Medical Device
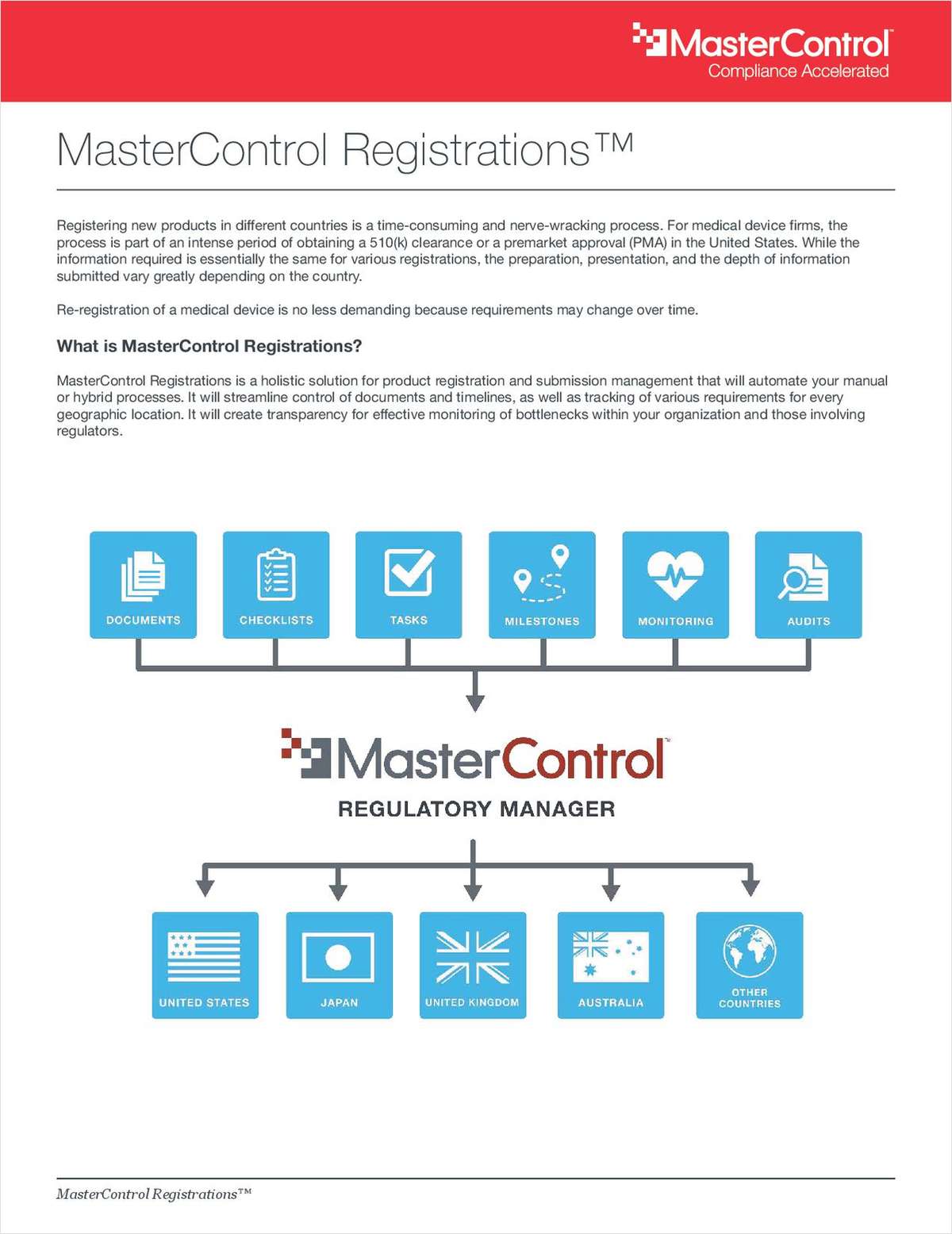
Registering new products in different countries is a time-consuming and nerve-wracking process.
For medical device firms, the process is part of an intense period of obtaining a 510(k) clearance or a PreMarket Approval (PMA) in the United States. While the information required is essentially the same for various registrations, the preparation, presentation, and the depth of information submitted vary greatly depending on the country. Re-registration of a medical device is no less demanding because requirements may change over time.





